
Micronutrient Management in the North Central United States & Canada
Micronutrients or minor elements are required by plants in very small quantities, usually less than a pound per acre per year. This does not diminish their importance. Most micronutrient deficiencies or toxicities are not widespread, but when they occur, plant abnormalities, reduced growth, or crop failure can result. Toxicities can also occur since several of these elements have rather narrow ranges between deficiency and toxicity levels. Once harmful levels have been established, the problem is more difficult to correct than a deficiency. This danger is greater on coarse-textured sands than on heavier soils.
In the past several years, increased interest has been shown toward the micronutrients due to (1) accumulation of more information regarding needs and plant responses; (2) induced deficiencies from higher crop yields; (3) greater removal from long-time cropping; (4) greater concern over crop quality and nutritional value; and (5) attempts by agribusinesses to maintain profit margins by increasing micronutrient use. In Wisconsin, particular attention has been drawn to foliar application of micronutrients to soybeans and the use of micronutrients on organic soils.
Basic Micronutrient Chemistry
In some cases, the micronutrient anions (boron, molybdenum, and chlorine), and cations (copper, zinc, manganese, nickel, and iron) have rather complex chemical relationships controlling their availability in soils. Overall, however, the relative availabilities are controlled by the equilibria that exist between the soil solution, the soil organic matter, the cation exchange sites, and insoluble compounds as illustrated.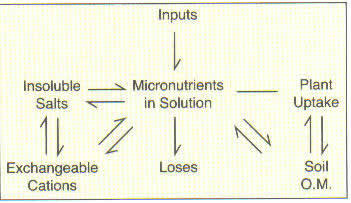
Organic Matter
The soil organic matter acts as a "storehouse" for many of these elements, especially the anions such as boron, which are subject to losses. As the organic matter decomposes, the nutrients are released and thus the organic matter tends to act as a continuous nutrient supply. Soils that receive regular additions of organic residues such as manures rarely, if ever, show micronutrient deficiencies.Soil Acidity or pH
The availability of almost all the micronutrients is affected by soil pH for several reasons. As soils are made more neutral, a better environment is created for microbial activity. This tends to stimulate the release of micronutrients from organic matter. The major influence, however, is the effect of pH on compound solubility. For all of the cations (copper, zinc, manganese, nickel, and iron) solubility is greatest under acid conditions. Boron availability also declines with increasing pH. The reverse, however, is true for molybdenum, perhaps due to the release of molybdenum from anion exchange sites or the conversion of molybdenum oxide to molybdenum salts, which is favored in alkaline soils.Soil Moisture Content
Most of the micronutrients can exist in several oxidation states, and in most instances, these nutrients tend to be more available in the reduced form. Waterlogged soils create reducing conditions so in some cases micronutrient toxicities occur in wet areas. In other situations, waterlogging may eventually result in deficiencies because of first increased solubility and then subsequent leaching of the more soluble form.
Boron deficiency is accentuated under dry soil conditions. This may be related to the rate of organic matter decomposition or rate of root proliferation.
Presence of Other Ions
Some studies have indicated that the absolute levels of some micronutrients in soils may not be the most important factor in relation to plant growth. The relative amounts of these elements in relation to one another may be more critical. Copper uptake, for example, is affected by levels of iron, manganese, and aluminum in the soil and copper levels, in turn, can affect zinc, iron, and phosphorus uptake. Other examples include reduced iron uptake by high levels of phosphorus, calcium, bicarbonate, or magnesium and ferrous iron can interfere with manganese uptake and high levels of sulfate with molybdenum uptake, perhaps due to ion competition at the root surface.
Weather
Weather conditions may influence micronutrient supply primarily through the influence on nutrient absorption. Cool, wet spring weather may retard the uptake of zinc and manganese. Usually when the temperature increases and the soil dries out, plants will grow out of the deficiency. As mentioned previously, dry weather tends to increase boron problems.
Crop Needs for Micronutrients
For all crops, the need for micronutrients in pounds per acre is small; however, relative needs vary from crop to crop and even between cultivars within a crop species. Table 1 indicates the relative micronutrient requirements for corn, soybean, small grains, and alfalfa. These interpretations generally mean that for soils that have low levels of those elements with medium or high needs, the chance is quite good that a yield response will be obtained if the nutrient is added. Iron deficiency has not been observed on field crops in Wisconsin and chlorine is typically not in short supply in field situations.

Occurrence of Micronutrient Problems
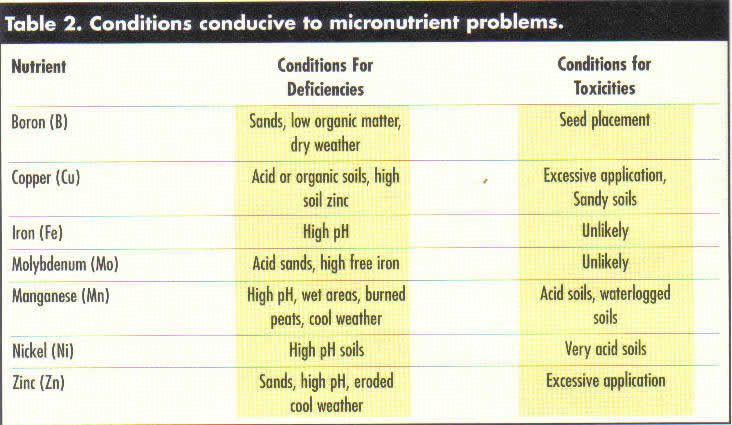
Considering the basic chemistry of the micronutrients, it is possible to predict the types of conditions and soils where micronutrient problems are more likely to occur (Table 2). As can be seen, the anionic micronutrients tend to be in short supply in those areas where they are readily leached and not being replenished by organic matter decompositions. Chlorine deficiency has rarely been observed east of the Mississippi River and never where KCI is commonly used. The cationic micronutrients are most likely deficient on calcareous soils or soils high in organic matter where strong chelation decreases availability.
Identifying Problem Areas
Visual Symptoms
Although it is often too late for corrective action by the time visual symptoms appear, this identification practice can be extremely helpful in planning for future years and in locating trouble spots in fields. It is often the first indication that a problem exists. The Ontario Soil Fertility Handbook (Publication 611) presents an excellent description of commonly occurring micronutrient deficiency symptoms for many crops.
Soil Tests
Soil tests are available for zinc, boron, copper, and manganese. At the present time, soil tests for iron and molybdenum have not been developed for use in most states, and are considered to be of little value in predicting the supply of these nutrients in soils. Since interpretations are soil specific, it is best to use locally calibrated recommendations.
Plant Analysis
Plant tissue analysis is a more reliable diagnostic tool than soil testing for identifying many micronutrient problems. All of the essential elements except the structural elements carbon, hydrogen, and oxygen, and molybdenum and chlorine can be determined by this method. Routine analysis of molybdenum is difficult and chlorine is usually sufficient under field conditions. This technique is especially valuable for those elements where no reliable soil test is available. Tissue analysis will also detect nutrient uptake interferences or show toxicities when a soil test alone is inconclusive. Plant composition varies with age, part of the plant sampled, and many other factors. Therefore, it is important to follow standard sampling procedures and to seek advice in interpretation of results.
Correction of Micronutrient Problems
Once a micronutrient problem has been confirmed, the best approach is to use the proper nutrient to correct it. It is not recommended to make broad-spectrum applications of these elements as this approach may be expensive and dangerous. The danger arises because all the nutrients in the mix may not be needed. As was noted previously, the presence of a second or third element may actually make the situation worse by interfering with the uptake of the needed nutrient. Note also that a mix may be relatively expensive because nutrients are being supplied that may not be needed.
 Timely application is essential. Experience has shown that by the time deficiency symptoms appear, irreversible yield losses have occurred. Apply the needed micronutrient prior to or at the time of planting. Also the benefits of application are directly proportional to the uniformity of the application. Spotty, uneven application patterns cannot be expected to provide uniform recovery and good plant growth. Considerable attention should be given to achieving a uniform spread pattern regardless whether the material is liquid or solid, banded or broadcast, applied preplant or foliar.
Timely application is essential. Experience has shown that by the time deficiency symptoms appear, irreversible yield losses have occurred. Apply the needed micronutrient prior to or at the time of planting. Also the benefits of application are directly proportional to the uniformity of the application. Spotty, uneven application patterns cannot be expected to provide uniform recovery and good plant growth. Considerable attention should be given to achieving a uniform spread pattern regardless whether the material is liquid or solid, banded or broadcast, applied preplant or foliar.
The method of application can have an important effect on the efficiency of the treatment. Relatively small applications of expensive material may indicate that banding or foliar applications are necessary. Because of the capacity for most soils to fix manganese very strongly, this material should only be banded. Some research has shown that this technique may also be preferable for zinc, but in other cases broadcast applications at slightly higher rates did equally well. Boron, on the other hand, should not be banded as high concentrations near the seed can be toxic. Foliar application is especially useful for some elements such as iron, which is not usually efficiently used when applied to the soil. Foliar applications are also useful for quick uptake in emergency situations or where other materials are being sprayed. However, as these applications are made after the crop has partially developed or in response to indicated needs, damage may have already occurred prior to the application, and more than one application may be needed.
Applications of molybdenum are normally measured in terms of ounces per acre. To achieve even application of these small amounts, seed treatment with the carrier may be the best technique available. This will place the nutrient where the plant can use it early in the growing cycle.
Recommended rates of micronutrients are highly specific for crop and method of application and, therefore, are not presented here. In general, since foliar and banded applications are somewhat more efficient in fertilizer use, these rates are proportionally lower. For more information on specific micronutrients, see the individual recommendations for your state or province.
Presented at the llth Annual Southwest Agricultural Conference held at Ridgetown College
References